Frequently Asked Questions about Molten Salt Reactors
Frequently Asked Questions
Q: What is thorium and what makes it special?
A: Thorium is a naturally-occuring mineral that holds large amounts of releasable nuclear energy, similar to uranium. This nuclear energy can be released in a special nuclear reactor designed to use thorium. Thorium is special because it is easier to extract this energy completely than uranium due to some of the chemical and nuclear properties of thorium.
Q: What is a liquid-fluoride reactor?
A: A liquid-fluoride nuclear reactor is different than conventional nuclear reactors that use solid fuel elements. A liquid-fluoride reactor uses a solution of several fluoride salts, typically lithium fluoride, beryllium fluoride, and uranium tetrafluoride, as its basic nuclear fuel. The fluoride salts have a number of advantages over solid fuels. They are impervious to radiation damage, they can be chemically processed in the form that they are in, and they have a high capacity to hold thermal energy (heat). Additional nuclear fuel can be added or withdrawn from the salt solution during normal operation.
Q: Are the salts safe?
A: Very safe. Unlike other coolants considered for high-performance reactors (like liquid sodium) the salts will not react dangerously with air or water. This is because they are already in their most stable chemical form. Their properties do not change even under intense radiation, unlike all solid forms of nuclear fuel.
Q: Have liquid-fluoride reactors been built before?
A: Yes, two liquid-fluoride reactors were built at Oak Ridge National Laboratory in Tennessee in the 1950s and 1960s. These were small research reactors that were built to test the fundamental principles of a liquid-fluoride thorium reactor. The first, which was called the Aircraft Reactor Experiment (ARE) ran for a week in 1957, and the second, the Molten-Salt Reactor Experiment (MSRE) ran between 1965-1969 and validated many of the principals of the fluoride reactor concept.
Q: How does a liquid-fluoride reactor make electricity?
A: Fission reactions take place in the fuel salt, making it hotter. This heat is transferred to a coolant salt outside of the reactor. The coolant salt is then used to heat gas that turns a turbine, which turns an electrical generator, generating electricity.
Q: What is nuclear waste and how does a liquid-fluoride reactor address this issue?
A: So-called “nuclear waste” or spent-nuclear fuel is produced in conventional (solid-core) nuclear reactors because they are unable to extract all of the nuclear energy from their fuel before they have to shutdown. LFTR addresses this issue by using a form of nuclear fuel (liquid-fluoride salts of thorium) that allow complete extraction of nuclear energy from the fuel.
Q: What advantages does a liquid-fluoride thorium reactor offer a utility?
A: Unlike a pressurized-water or boiling-water reactor, a liquid-fluoride thorium reactor operates at high temperature and low pressure. Its high power density means that the reactor vessel itself is much s maller and lighter than an LWR reactor vessel; small enough, in fact, to be mass-produced in a factory rather than constructed onsite. Its inert-gas coolant does not boil in the event of a loss of pressure, and the fuel, blanket, and coolant salts do not react with air or water. All of this means that the containment building of a fluoride reactor can be much smaller than the containment of a light-water reactor of similar power output.
Q: What’s the difference between a “fast-spectrum” reactor and a “thermal-spectrum” reactor?
The basic idea behind nuclear fission is that you can use an electically neutral particle, the neutron, to destabilize a nucleus and cause it to split. This is a big deal because it’s very difficult to get charged particles, like protons and electrons, anywhere near the nucleus–they’re repelled by electrical forces. That’s the basic reason why nuclear fusion is so difficult.
But with the neutron, it’s a different story. It just waltzes right up to a nucleus and hits it, and the nucleus never saw it coming.
Here’s an animated gif of how fission works, and a little movie too.
Now the speed of the neutron when it hits the nucleus has a lot to do with how likely a fission is to occur. One might think, intuitively, that if the neutron is going really fast that it has a better chance of “shattering” the nucleus, but that’s not really how it works. Actually, for the fissile nuclei (such as U-233, U-235, and Pu-239) the SLOWER the neutron is going, the more probable fission is.
So you want slowed-down neutrons to maximize fission. And then from fission comes more neutrons, which continue the reaction. Well, mostly right. Actually, the neutrons borne from fission are going really fast. Really, really fast. And they have to slow down to have a good chance of causing fission. That’s where the moderator comes in.
The moderator in a nuclear reactor is the material whose job it is to slow down neutrons without absorbing them. This slowing-down is done by neutrons bouncing off the nuclei of the atoms in the moderating material. For most reactors, moderation takes place in the water that also cools the reactor. For a high-temperature reactor like the liquid-fluoride reactor, graphite (carbon) is used as the moderator.
The neutrons are born from a fission reaction, bounce around in the moderator, slow down, and then cause another fission reaction. This “bouncing-around” process is also called “thermalizing” the neutrons, because by bouncing around in the moderator, the neutrons are brought to the point where they have the same thermal energy as the surrounding material.
This graph shows how likely a fission reaction is based on the speed (kinetic energy) of the neutron that strikes the nucleus is. Cross-section is a concept that corresponds to the probability of interaction–the larger the cross-section, the more the probability of interaction. The energy of the thermalized neutron corresponds to temperature. If a neutron were at the same temperature as the room you’re in (~300 K), it would have an average energy of 0.025 eV. Not very much. If the neutron instead were at the same temperature as the hot fluoride salt in the center of a liquid-fluoride reactor (~1000 K) its average energy would be 0.086 eV. Not much more.
When neutrons are born from the fission reaction, they have energies around 2,000,000 eV, which corresponds to a temperature of 20 billion degrees! That’s much hotter than the center of the Sun! But like hot water poured into snow, when neutrons are that much hotter than their surroundings, they lose energy fast. And most all of that energy is lost through collisions with the nuclei of the moderating material.
So a “thermal-spectrum” reactor is a reactor that has been arranged in such a way so as to optimally “cool” the neutrons so they can cause fission. And as can be seen from the graph, fission is hundreds of times more likely when neutrons are “cooled” down by thermalization/moderation than when they’re “fast”.
So it’s logical to ask at this point, why would anyone want to build anything but a thermal-spectrum reactor? It would seem to have the minimum amount of fuel requirement for a reactor, and it would seem to maximize your chances of getting nuclear reactions. And indeed it does. But there is more to the story.
Uranium is an interesting substance, consisting overwhelmingly (99.3%) of an isotope, uranium-238, that is not fissile. But if uranium-238 captures a neutron it becomes plutonium-239, which is fissile. One more neutron into the plutonium and you get a fission reaction and energy. So you can imagine that it takes two neutrons to “burn” uranium-238.
But there is a very small amount of uranium (0.7%) that consists of the isotope uranium-235, which is fissile and only requires one neutron to fission. Despite constituting such a small fraction of uranium, this U-235 is where nearly all of our nuclear energy comes from today. And the fact that we are burning up this small resource is one of the basic reasons that our nuclear infrastructure is not sustainable. It’s also one of the basic reasons that today’s reactors make so much nuclear waste.
So couldn’t we just burn up the U-238 after the U-235 is gone? Well, to do that, we need to make sure that the fission of Pu-239 (which is what U-238 turns into after it absorbs a neutron) gives off at least two neutrons–one to convert a new U-238 into Pu-239, and another to fission that Pu-239. So how many neutrons does the fission of Pu-239 give off? Well, it all depends on the energy of the neutron that the Pu-239 absorbs. Here’s a graph showing the relationship.

Now this graph shows two lines. One is the line in purple that shows how many neutrons are given off from a fission in Pu-239. As you can see, it’s pretty constant across energies–nearly three neutrons emitted per fission. That seems to indicate there will be plenty of neutrons for fission, conversion, and even some to spare. But the blue line tells a different story. The blue line is the number of neutrons given off per absorption in Pu-239. Why are they different? Because Pu-239 has the unpleasant habit of sometimes just absorbing the neutron that struck it, and not fissioning. This happens more often when the neutron it absorbs is at the slowed-down, thermal energies.
The fact that plutonium-239 likes to eat thermal neutrons and not fission has tremendous implications for our energy future. At thermal neutron energies, the effective number of neutrons given off per absorption isn’t enough to sustain “burning” of U-238. You can see the line dip and weave around the magic 2.0 number at thermal energies (the energies at the left-hand side of the plot). When you account for neutron losses and a number of other things that real reactors must deal with, there’s just not enough neutrons to go around.
Here is the point where the road forks, where two paths present themselves, and one was taken, and the other effectively ignored. One path is thorium, the other path is the plutonium fast-breeder.
The path that was taken, or at the very least, the path that the nuclear community has wanted to take for the last sixty years, is the path to the plutonium fast-breeder. Confronted with the data that you can’t get enough neutrons from a thermal-spectrum reactor to “burn” U-238, they began to investigate what happens if you use a “fast-spectrum” reactor. At “fast” energies (the energies on the right-hand side of the plot) things start to look a lot better for plutonium. It makes significantly more neutrons per absorption than 2, and so the “burning” of U-238 looks to be quite feasible. But now you have a different problem, that of building a fast-spectrum reactor.
But before I go too far, let’s talk about the path not taken–thorium. Thorium is about three times more common than uranium and consists of only one isotope, thorium-232. It has no naturally fissile isotope like U-235, and thorium is not fissile in and of itself. But like U-238, it can be converted into a fissile isotope (U-233) by absorbing a neutron. One more neutron absorption in U-233 causes fission. So again, we ask the question, how many neutrons does the fission of U-233 give off? Is it more than 2? More to the point, is it more than 2 per absorption?
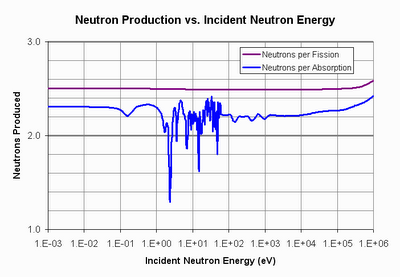
Yes, U-233 not only gives off more than two neutrons per absorption at thermal energies, it gives off significantly more than 2, which is enough to account for the inevitable losses that will occur in a real reactor. This means that a thermal-spectrum reactor can “burn” thorium in a sustained manner and doesn’t need to go to a fast-neutron spectrum. And that has tremendous advantages for safety, economy, and nuclear proliferation.

Thanks for a neat webpage with a lot of info – hightening the info is the path to the future.
Keep up the good work.
BR Mogens
I have been studying Molten Salt Thorium reactors for years now. The whole process is amazingly simple and it is a perfect answer to the world’s energy needs. It is actually 4 X the abundance of U235 which is only 0.7% the abundace of all mined uranium! So small it can be ignored. Everyone has expressed corrosion resistance problems but the whole world has moved on in metallurgy and ceramic materials since then. In fact silicon carbide ensures that fission products in pebble bed reactors STAYS in the pebble. The US threw away the advantage of Thorium reactors because they couldn’t blow shit up with it. China meanwhile researched Thorium from the word go because Thorium is a resource they have in abundance. When you mine for rare earth elements vital for modern electronics and magnet technologies, thorium comes up with and at the moment is regarded as waste rock from the process. It reminds me that Mdme Curie obtained radium from Pitchblende which was brought up at a Silver mine in Chechoslovakia and comes from the old Czech word’s of Pic and Blende, meaning, “Shit Rock” suggesting that history is repeating itself!