Energy is Essential for Life
The generation and use of energy is central to the maintenance of organization of civilization. This is an immutable principle of thermodynamics. Life itself is a state of organization maintained by the continual use of sources of energy. For millennia, humanity has been using more and more energy to attain a higher standard of living.

In all cases, human society has been moving from low-density energy sources to higher-density energy sources. This trend has been present for thousands of years. The earliest human communities relied on the energy of a single individual, to hunt, to plant, to harvest. The energy harvested from the hunt or the planting was partially consumed in the search for the next day’s energy. But the domestication of animals brought a great advance in energy utilization, since large, useful animals consumed grasses and leaves that humans could not eat, and their strength could pull the plow that turned the earth or carry the hunter to his quarry. Domestication of animals led to widespread agriculture and the first civilizations in the ancient world. It was a substantial improvement in energy density.


For thousands of years the only energies available for work were those of man and his animals, but in some cases people found natural flows of energy that could be harnessed. These might be flowing water or a steady wind, but they were put to use to solve the most pressing needs of the day, grinding wheat or pumping water so that civilization might survive a bit longer.

Fossil fuels like coal and petroleum had been known since ancient times. They could be burned for heating but so could firewood or agricultural waste. An incredible technological advance took place in the 1700s when, for the first time, a practical means was devised for transforming thermal energy into useful work: the steam engine.

It is difficult to overestimate the effect of the steam engine on the progress of humanity, but consider how limited the options were for turning energy into work before the steam engine. One could feed animals or humans and use them as power conversion systems, but this was limited to the slow rate of digestion and the endurance of living creatures. One could find the rare locations where natural energy flows could be harnessed, such as wind and water flows, but these were intermittent and limited in energy density.

The steam engine changed all that, since it was the first device that could transform thermal energy into mechanical energy (or work). Such a thing had never existed, and since it was relatively easy to generate thermal energy by burning things, with the steam engine work could be generated in all kinds of locations that before had been utterly impractical. One of the first places steam engines were used was in mines, to pump out water from deep within the mine. This increased the amount of coal that could be mined, and this coal in turn fed the steam engines of the day.
Another early application of steam engines was to use the mechanical work they generated to turn a wheel and to propel a vehicle. The first steam locomotives were introduced in the 1830s, and burning wood and later coal, they revolutionized transportation. For all of human history, the fastest man could travel was on a horse, but soon steam locomotives were reaching speeds of sixty miles per hour or greater. They did not tire like a horse but required large amounts of fuel and water to operate. The demand for rail travel increased the demand for iron, steel, brass, and production increased tremendously.
The entire science of thermodynamics developed from the insights of scientists and engineers around the steam engine, and the other “heat engines” that would follow it. One of the fathers of thermodynamics was a young French military engineer named Sadi Carnot. Carnot did not invent a better steam engine, rather he mused on how efficiently a steam engine could possibly operate. In 1824, when Carnot was only 28, he published his book \emph{Reflections on the Motive Power of Fire}, where he describes his realization that there was a limit on how much of the thermal energy of fire (or any other kind of heating process) could be converted into mechanical work. Lord Kelvin, born the same year Carnot published his book, would summarize Carnot’s realizations into a simple equation:
![]()
For engineers, this equation had tremendous value, for it described the direction they should move in order to improve the steam engine, or any other kind of mechanical engine based on heating. The key was to heat the working fluid of the engine (be it steam or air) at the highest temperatures possible. The temperature at which water boils is proportional to the pressure it is under, with water boiling at higher and higher temperatures under greater and greater pressures. Thus, the key to improving the efficiency of steam engines was to boil water within the engines at higher pressures. Higher temperatures and pressures required better and stronger materials, and this required a better understanding of matter itself, driving advances in chemistry, metallurgy and materials science.
With the steam engine and its ability to convert thermal energy into mechanical energy, greater interest was focused on the ability to convert mechanical energy into electrical energy. Beginning with Michael Faraday’s work at the Royal Institution in 1821 on simple motors and culminating in Nikola Tesla’s work in 1888 on electric induction motors, scientists and engineers successfully perfected machines that could transform mechanical energy into electrical energy. The profound advantage of electrical energy was the ease at which it could be transmitted over distances far greater than mechanical energy.
One of the less-appreciated accomplishments of the development of electrical motors and the advances in chemistry was the production of aluminum metal. Aluminum is the most abundant metallic element in the Earth’s crust, but it is so tightly chemically bound to oxygen that it never occurs as a free metal; rather, it is always found as a ceramic material, aluminum oxide (Al2O3). Only one chemical was powerful enough to tear aluminum away from oxygen, and that was fluorine. But fluorine’s extreme reactivity also meant that it was never found free in nature; rather, it was always tightly bound to other metals, most commonly with calcium. Calcium fluoride was called fluorite or fluorspar, and when combined with sulfuric acid at high temperatures produced hydrofluoric acid (HF) and gypsum (CaSO4•2H2O). Gypsum was used for plaster while the HF was used to react with aluminum oxide and sodium fluoride to form a material called cryolite (Na3AlF6). Cryolite was a sodium-aluminium-fluoride salt that melts at a temperature of approximately 1000°C. But with the development of electrical generation from early electric motors, aluminum metal could be isolated from cryolite using a process called the Hall-Heroult process, discovered in 1886 independently by an American and a Frenchman. The Hall-Heroult process requires large amounts of electrical energy to split off the aluminum metal from the cryolite, but its development was one of the most tangible benefits of the development of electricity.
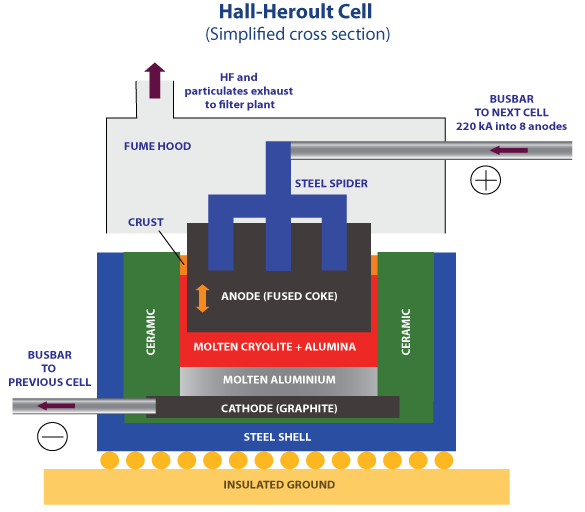
Aluminum metal was light and strong and soon in high demand. Soon aluminum metal had been transformed from one of the most expensive substances on earth to one of the least expensive.
The internal combustion engine was another class of engine that used air as the working fluid rather than steam. It became a practical alternative to the steam engine when purified liquid fuels became available that could be atomized and spraying into an internal combustion chamber. It represented a significant simplification over the steam engine since it used the atmosphere as the working fluid rather than requiring continuous addition of water like the steam engine. Unlike the steam engine, however, it relied on a much more limited fuel supply. Coal and firewood could not be used in an internal combustion engine; only a purified liquid fuel was suitable.
Petroleum was an even better energy source than coal, since it could be refined and combusted directly with a minimum of soot and ash. The intermediate step of boiling water for steam could then be removed and internal combustion and gas turbine engines became possible that were much more compact and power-dense.
Aluminum metal could be fabricated into internal combustion engines that were light enough to power aircraft, and it is the availability of metallic aluminum that played a large role in the advent of air transportation. Air travel relied on liquid fuels, increasing demand for refined petroleum products. The incredible energy density of liquid hydrocarbon fuels from petroleum, coupled with the incredible power density of reciprocating and gas-turbine engines, truly made a modern mobile lifestyle possible.
As revolutionary as the steam engine had been, enabling the thermal energy of fire to become the mechanical energy of work and motion, another revolution was coming that had the potential to be even greater. Ever since Marie Curie and her colleagues had discovered radioactivity in the late 1800s,
But in 1938 a discovery was made that was probably the historical equivalent of the discovery of fire, and it was simple—that there was an entirely new world of energy located in the nucleus of the atom itself. Up until this point, everything we had done with energy had to do with the electrons in the atom. We changed their configuration through digestion, combustion, and explosion, but we never touched the world of the nucleus. It remained, isolated and untouched by the outside world, until our understanding of the nature of matter and energy allowed us to reach out and touch it. Then and now, the instrument with which we use to touch the nucleus and unlock its incredible energies is a piece of the nucleus itself: the neutron.
\begin{figure}[htp]
\begin{center}
\includegraphics[width=0.4\textwidth]{images/atom-hidden-heart.jpg}
\end{center}
\end{figure}
and there is no reason to think that this trend has reached an end.
We live in a time when it has become fashionable to make negative associations around the use of energy. We are told to use less, turn it off, conserve. Waste is never a virtue, but we should not lose sight of what a glorious achievement the widespread use of energy has been for human civilization.
Therefore, we should embrace the idea that we need energy and ask ourselves which characteristics we would desire for energy generation. This statement should not be construed so as to imply that we should waste energy or avoid ways to improve the efficient use of energy, but that we should seek to generate energy in ways that are reliable, compact, resilient, and environmentally sound.
